
Hydrogel Alternative: FLEXcon Creates a Healthcare Industry Disrupter
Fast forward to 1974, to a church where Silver’s colleague, Art Fry, was singing in a choir. Fry was frustrated because the bookmarks he was using in his hymnal kept falling out. Suddenly, he remembered Silver’s project. He went back to 3M and applied the adhesive to small pieces of paper that could be used as bookmarks. The Post-it® Notes was born.
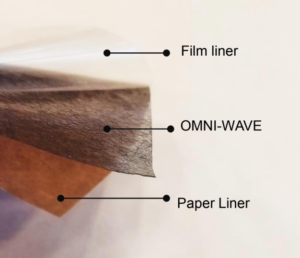
History is filled with these types of discoveries that happen by accident. Such is the case with FLEXcon® OMNI-WAVE™, a new product that’s been developed by FLEXcon as an alternative to hydrogel.
Initially started as a solution for the battery sector, it’s now poised to disrupt a medical industry standard, and in the process, provide welcome relief to patients and converters alike. According to Aditi Subramanian, a Healthcare Business Unit Manager for FLEXcon, “It’s a true case of iteration and innovation.”

Hydrogels: Helpful, yes, but with drawbacks to patients and medical practitioners
Hydrogels have been around for over 50 years. They’ve been used in the medical industry both for diagnostics (to pick up and receive a signal which is then filtered) and therapeutics (using low-voltage electric currents to treat pain).

The most common use is on an electrocardiogram electrode. The hydrogel, a water-based solution that is applied to the skin, acts as a barrier to the monitoring electrodes.
Hydrogels have been instrumental in helping doctors monitor patients for years, but they do have a number of problems, including:
- They can cause adverse skin reactions. Estimates are that up to 8% of people have allergic reactions to hydrogels, including mild rashes and welts. With neonatal care, infants can experience large blisters.
- They are quick to dry out. As they’re water-based, anytime a package is opened, the material starts to dry out, limiting the strength of the signal. That also dramatically shortens the product’s shelf life.
- They are expensive. As the only game in town, medical practitioners have no alternatives to hydrogels and are forced to pass the high cost of the product on to the patient.
- They don’t work when moist. Hydrogel, as the name suggests, is based on water as a component. When moist (sweat or humid environment), the hydrogel layer loses its effectiveness.
The high costs of hydrogels are a result of how difficult it is for the converting industry to produce the product, a pain many of our converters feel every day.
Converters struggle with hydrogel production process and costs
No one ever said work in the converting industry would be easy. At Delta ModTech, we and the converters we partner with pride ourselves on tackling new challenges. But the production issues involved with hydrogel are particularly troublesome.
“100% of the medical converting community probably hates processing hydrogel,” Subramanian said. “Converters know it’s a massive pain to work with.”
Typical converting issues with hydrogel include:
- Problematic to produce. Because the gel material is 20-40 mil thick, it’s messy and often gums up the die-cutting tool.
- Heavy rolls, difficult to maneuver and ship. Hydrogels come in 300-foot rolls, and weigh in around 75 pounds. That results in some significant shipping costs. They’re also difficult to change on the press, adding downtime to the process, and are hard to hold on a release liner.
- Expensive packaging. From a production standpoint, producing a packaging barrier that retains the water is expensive.
All these hydrogel headaches have been endured for years. But FLEXcon didn’t set out to help converters and the medical world when it developed OMNI-WAVE™. It had another industry in mind.
Borrowing from the battery sector
FLEXcon’s product was not created to replace hydrogels. Like Spencer Silver, they were working on an application for a different use case — batteries.
The FLEXcon team’s goal was to develop an adhesive with ductility. Upon completion of the prototype, they realized that their solution may have reached beyond battery applications, functioning similarly to hydrogels in the patient care space.
OMNI-WAVE™ features new material that embeds electrodes in a thin, adhesive carbon veil. The material can be applied directly to a patient’s skin. “So long as it is touching the body, you are going to get a signal,” explained Subramanian.
The signal area is also significantly improved. In the graphic below, note the added hydrogel layer peeking out below the adhesive layer. It has a smaller signal area and requires an extra production step. OMNI-WAVE™ has a larger signal area and eliminates the need for an additional production step.

Beating testing standards designed for hydrogel
Because the hydrogel has been a long-time standard, FLEXcon had to use testing and independent research to prove they had a viable replacement.

ADITI SUBRAMANIAN, HEALTHCARE BUSINESS UNIT MANAGER, FLEXcon
“We had to work to show that we were equivalent or better than the standard.”
It has succeeded on multiple fronts, including:
AAMI compliant. AAMI has developed a test standard — EC-12 — that is basically geared for hydrogel. OMNI-WAVE™ passed EC-12 tests with ease.
Independent medical studies. According to two UMASS Medical School clinical trials, OMNI-WAVE™ electrodes were compared to Kendall ECG readouts and DOT ECG readouts and found to be essentially the same.
ISO 10993-10 approved. The product is ISO 10993-10 approved for sensitization and irritation.
In addition to the testing standards, OMNI-WAVE™ has over 13 US patents and 90 patents worldwide.
Innovize conducts a hard and soft cost comparison
Innovize, a contract manufacturing company with a focus on stick-to-skin medical devices, conducted a hard and soft cost comparison analysis between OMNI-WAVE™ and traditional hydrogel materials.
They found that while the cost of the materials are comparable, there were several advantages to OMNI-WAVE™ when costing products for customers:

Here are more details on the comparison.
Rollout geared to the pace of disruption
When you innovate to the degree that FLEXcon has with OMNI-WAVE™, you open up a whole new set of possibilities. But despite all the potential to replace hydrogel, FLEXcon understands that an application as potentially disruptive as OMNI-WAVE™ won’t be implemented overnight.
That’s why FLEXcon is rolling it out as a wearable product for up to 3 day use. This is more in-line with the trend to use wearable devices that communicate data to the cloud, and it’s a space in need of more innovative technologies.
As OMNI-WAVE™ becomes more widely used, FLEXcon hopes they’ll become more widely accepted in more established use cases.
But if you’re a converter or OEM looking for immediate relief from hydrogels production woes, there’s no reason why you shouldn’t be one of the first to trigger the disruption. Based on the data, medical practitioners and patients will thank you for it.
Learn more about OMNI-WAVE™ from FLEXcon!
Published on Jul 17 2023
Last Updated on Jul 22 2024
Categories: Delta ModTech Blog, Materials, Medical Devices, Reducing Scrap, Tapes / Gaskets
Next Post
Label Expo Europe
Previous Post
Delta Hosts Local Robotics Camp

